Laser-based system achieves noncontact medical ultrasound imaging

Researchers from MIT Lincoln Laboratory and their collaborators at the Massachusetts General Hospital (MGH) Center for Ultrasound Research and Translation (CURT) have developed a new medical imaging device: the Noncontact Laser Ultrasound (NCLUS). This laser-based ultrasound system provides images of interior body features such as organs, fat, muscle, tendons, and blood vessels. The system also measures bone strength and may have the potential to track disease stages over time.
"Our patented laser system concept seeks to transform medical ultrasound by overcoming the limitations associated with traditional contact probes — namely, the variability in technique among ultrasound technicians and the need to touch patients," explains principal investigator Robert Haupt, a senior staff member in Lincoln Laboratory's Active Optical Systems Group. Haupt and senior staff member Charles Wynn are co-inventors of the technology, with assistant group leader Matthew Stowe providing technical leadership and oversight of the NCLUS program. Rajan Gurjar is the system integrator lead, with Jamie Shaw, Bert Green, Brian Boitnott (now at Stanford University), and Jake Jacobsen collaborating on optical and mechanical engineering and construction of the system.
Medical ultrasound in practice
If your doctor orders an ultrasound, you can expect a highly trained sonographer to press and manipulate a handheld device (ultrasound transducer, also referred to as a probe), onto your body. As the sonographer pushes the probe across your skin, high-frequency acoustic waves (ultrasound waves) penetrate and propagate through your body tissue, where they "echo" off different tissue structures and features. These echoes come from changes in tissue strength (tissue softness or rigidity) from fat, muscle, organs, blood vessels, and bone deep inside the body. The probe receives the returning echoes, which are assembled into representational images of the body's internal features. Specialized processing is used to construct the shapes of the tissue features in 2D or 3D, and these constructions are then displayed on a computer monitor in real time.
Using ultrasound, doctors can noninvasively "see" inside the body to image diverse tissues and their geometries. Ultrasound can also measure blood flow pulsing through arteries and veins, and can characterize the mechanical properties (elastography) of tissues and organs. Doctors routinely use ultrasound to evaluate and diagnose a variety of health conditions, diseases, and injuries. For example, ultrasound can be used to image the anatomy a developing fetus, detect tumors, and measure the degree of narrowing or leakage in heart valves. Ranging from handheld devices on an iPhone to cart-based systems, ultrasound is highly portable, relatively inexpensive compared to other medical imaging technologies like magnetic resonance imaging (MRI) and computerized tomography (CT), and widely used in point-of-care and remote-field settings.
Limitations of ultrasound
Though state-of-the-art medical ultrasound systems can identify tissue features within fractions of a millimeter, the technique has some limitations. Because sonographers manipulate the probe with their hands to obtain the best viewing window into the body, ultrasound is prone to imaging errors. More specifically, as sonographers apply pressure to the probe by feel, they randomly compress the local tissue where the probe makes contact. This compression causes unpredictable changes in the tissue properties that impact the travel paths of the ultrasound waves. Ultimately, this compression distorts the resulting images of tissue features, with feature shapes not accurately plotted. In addition, tilting the probe, even slightly, changes the angle plane of the image view — skewing the image and creating uncertainty of where features are positioned in the body.
These limitations can be significant enough that ultrasound cannot resolve with sufficient confidence, for example, whether a tumor is getting larger or smaller and precisely where the tumor is located in the host tissue. Furthermore, the uncertainty in the size, shape, and position of tissue features will vary upon repeat measurement, even for the same sonographer trying to retrace their steps. This uncertainty, termed operator variability, is more severe when different sonographers attempt the same measurement, leading to inter-operator variability. Because of these drawbacks, ultrasound is often restricted from tracking cancerous tumors and other disease states. Instead, methods such as MRI and CT are mandated to track how diseases progress — even with their vastly higher cost (on the order of one million dollars), greater system size and complexity, and imposed radiation risk.
“Variability has been a major limitation of medical ultrasound for decades," says Dr. Anthony Samir, associate chair of Imaging Sciences at MGH Radiology and director of CURT. Samir and his MGH CURT colleagues Dr. Kai Thomenius and Dr. Marko Jakolvejic provide critical medical experience, technical expertise, and guidance on conventional ultrasound devices to the Laboratory team and collaborate with them on NCLUS system development.
By fully automating the process for acquiring ultrasound images, NCLUS has the potential to reduce the need for a sonographer and to mitigate operator variability. The laser positioning can be accurately reproduced, thus eliminating variability across repeated measurements. Because the measurement is noncontact, no localized tissue compression or its related distortion to image features occur. Moreover, similar to MRI and CT, NCLUS provides a fixed-reference-frame capability using skin markers to reproduce and compare repeat scans over time. To support such tracking capabilities, the Laboratory team developed software that processes ultrasound images and detects any changes between them. Requiring neither manual pressure nor coupling gels (as required by contact probes), NCLUS is also ideal for patients with painful or sensitive body areas, in fragile states, or at risk of infection.
"NCLUS could image burn or trauma victims, patients with open deep-tissue regions directly during surgery, premature infants requiring intensive medical care, patients with neck and spine injuries, and contagious individuals from standoff distances," Haupt says.
Light-induced ultrasound waves
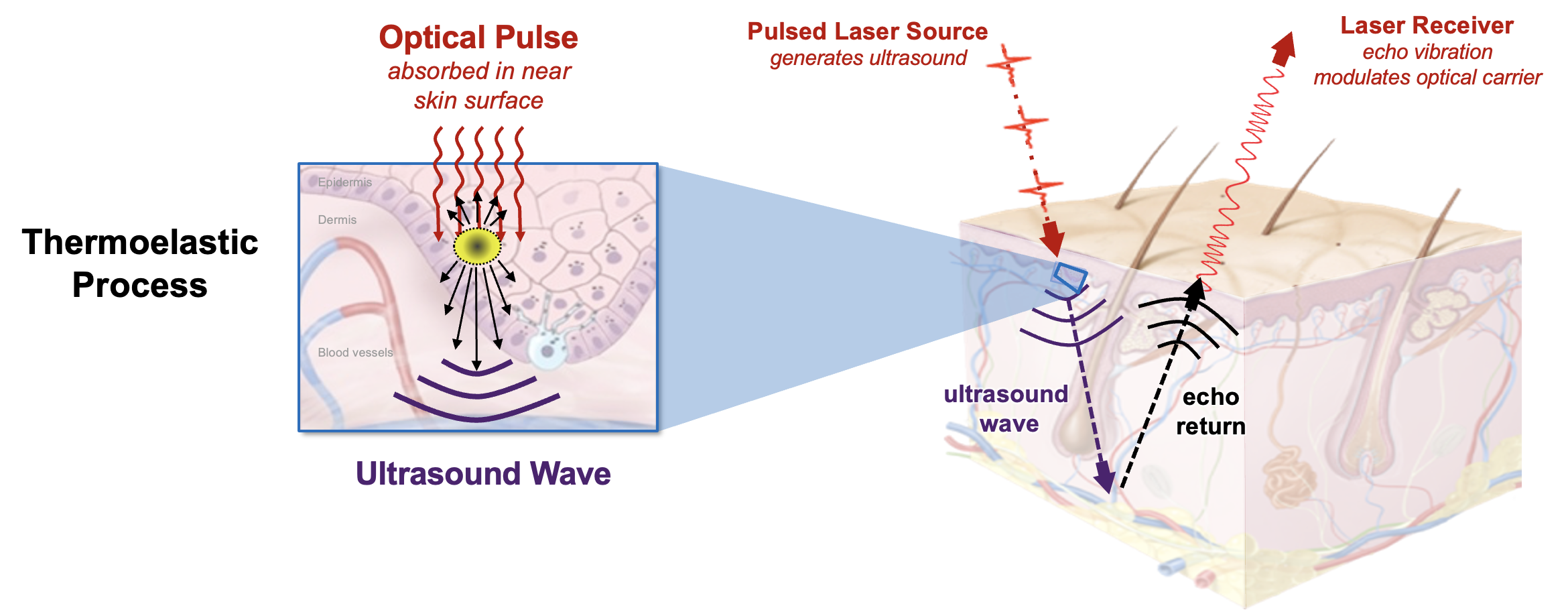
NCLUS leverages low-cost laser components. A pulsed laser transmits optical energy (at a level that adheres to laser safety standards for skin exposure) through the air to the skin surface, where the light is rapidly absorbed once in the skin. The optical pulse causes instantaneous localized heating and rapidly deforms the skin through a thermoelastic process that in turn generates ultrasonic waves, acting as an ultrasound source — a phenomenon called photoacoustics.
The optical pulse yields sufficient ultrasound power with frequencies comparable to that of practiced medical ultrasound while causing no sensation on the skin. The team patented the choice of the optical carrier wavelengths, which correspond to the wavelengths that water content in tissue most absorbs optical energy. The photoacoustic process is designed to create a consistent ultrasound source, independent of skin color or tissue roughness (e.g., caused by scar tissue, normal aging, or working environments such as the outdoors).
The ultrasound echoes returning from the tissue interior emerge at the skin surface as localized vibrations. These vibrations are measured by a laser Doppler vibrometer, a contactless sensor that uses beams of light as ultrasound receivers. The sensor exploits the Doppler effect, a physics phenomenon describing the change in frequency of a sound or light wave due to relative motion of the wave source or an observer. This effect explains why the pitch of a siren rises as an ambulance speeds toward you and then drops as the ambulance drives away, for example.
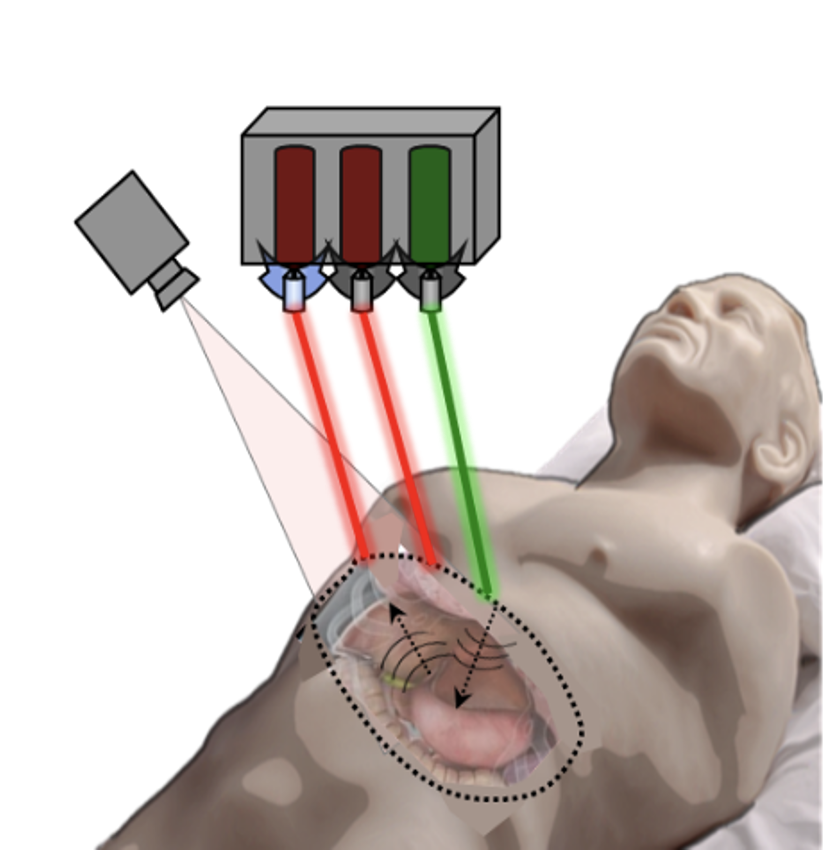
"By setting up the laser transmit (pulsed laser) and receive (laser vibrometer) components in the appropriate configuration, any exposed tissue surfaces can become viable ultrasound sources and detectors," Haupt explains. "In other words, the ultrasound array setup for NCLUS is highly versatile."
Advances toward a clinically operational system
In 2019, the team demonstrated that the NCLUS proof-of-concept (GEN-1) system can acquire ultrasound imagery from human subjects using skin-safe lasers — a first in the medical community. However, compared to practiced ultrasound, the GEN-1 system took significantly longer to acquire image data from the patient and was thereby impractical for clinical practice, where the flow of patients through a facility is key to operational efficiency. In addition, the GEN-1 system image resolution was significantly less than that of state-of-the-art medical ultrasound.
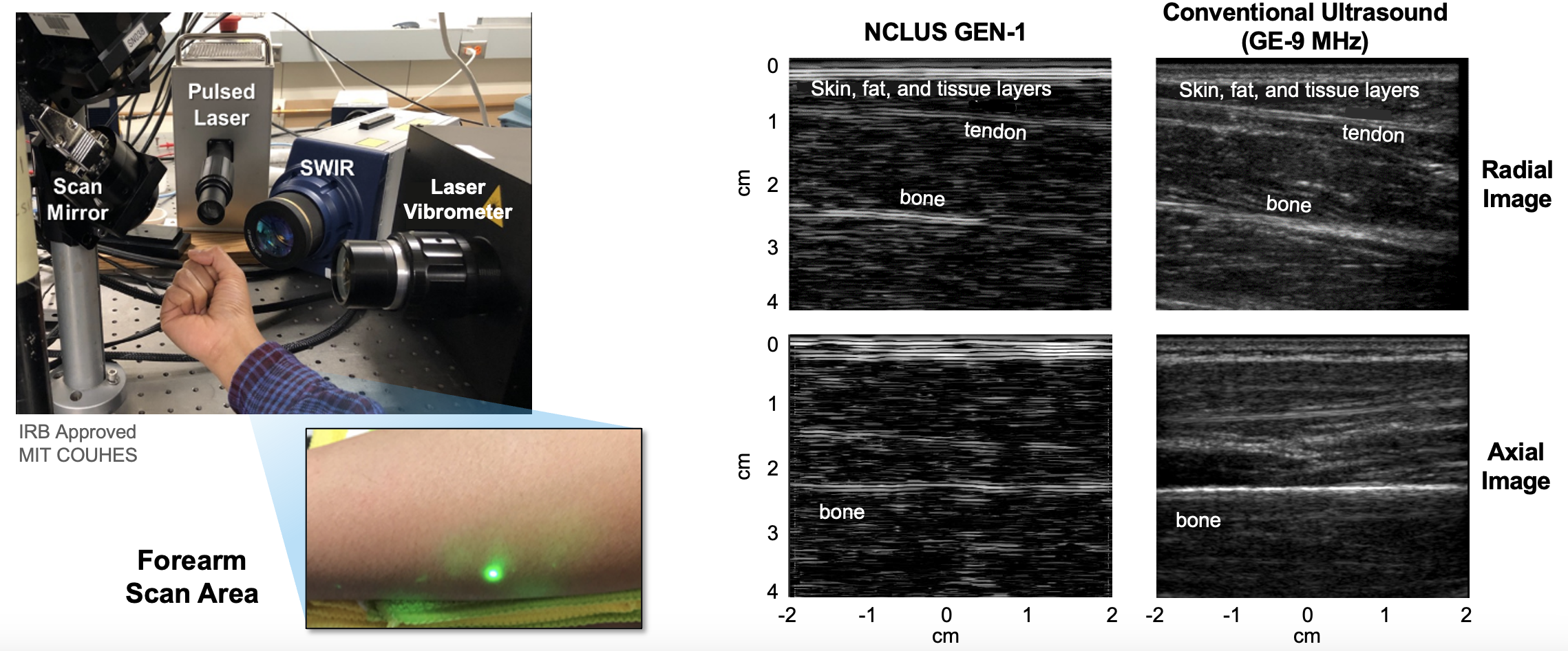
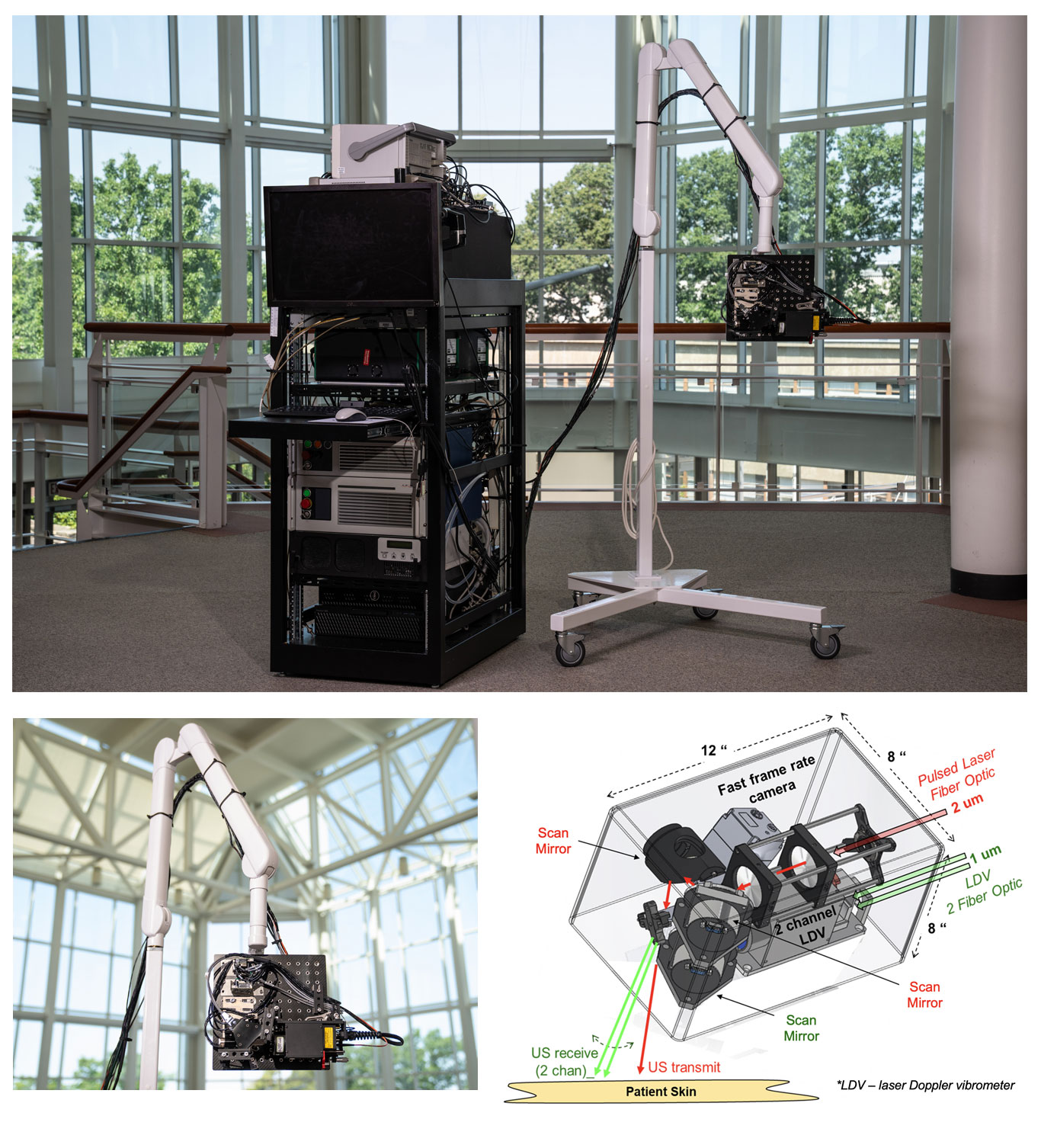
Significant engineering development has since occurred to transition NCLUS GEN-1 to an operational system appropriate for clinical testing. In the clinical NCLUS system, both the laser source and receiver are miniaturized and housed inside an optical head attached to a portable armature. The lasers that pulse and scan are 500 times faster than those of the GEN-1 system, thus reducing the entire image-data acquisition time to less than a minute. Future NCLUS prototypes will involve faster acquisition times of less than one second. The new clinical system also operates at much higher ultrasound frequencies than those of the GEN-1 system, enabling resolution down to 200 microns, which is comparable to the resolution of state-of-the-art medical ultrasound.
The moveable armature enables many degrees of freedom to view the various regions of the body. Inside the optical head are also programmable fast-steering mirrors that automatically position the source and receive laser beams to precisely establish the ultrasound array. A 2D lidar (which stands for light detection and ranging) is used to map the patient's skin surface topography by transmitting laser light at the skin and analyzing the reflected light; a high-frame-rate camera sensitive to energy in the short-wave-infrared (SWIR) wavelength range records the laser source and receiver projected locations on the skin, providing the array parameters necessary for constructing ultrasound images. The skin-surface topography mapping and laser-position recordings are registered by using natural skin features such as freckles, birthmarks, or scars. In this way, a fixed reference frame is established for performing precise repeat scans over time, for any skin pigmentation.

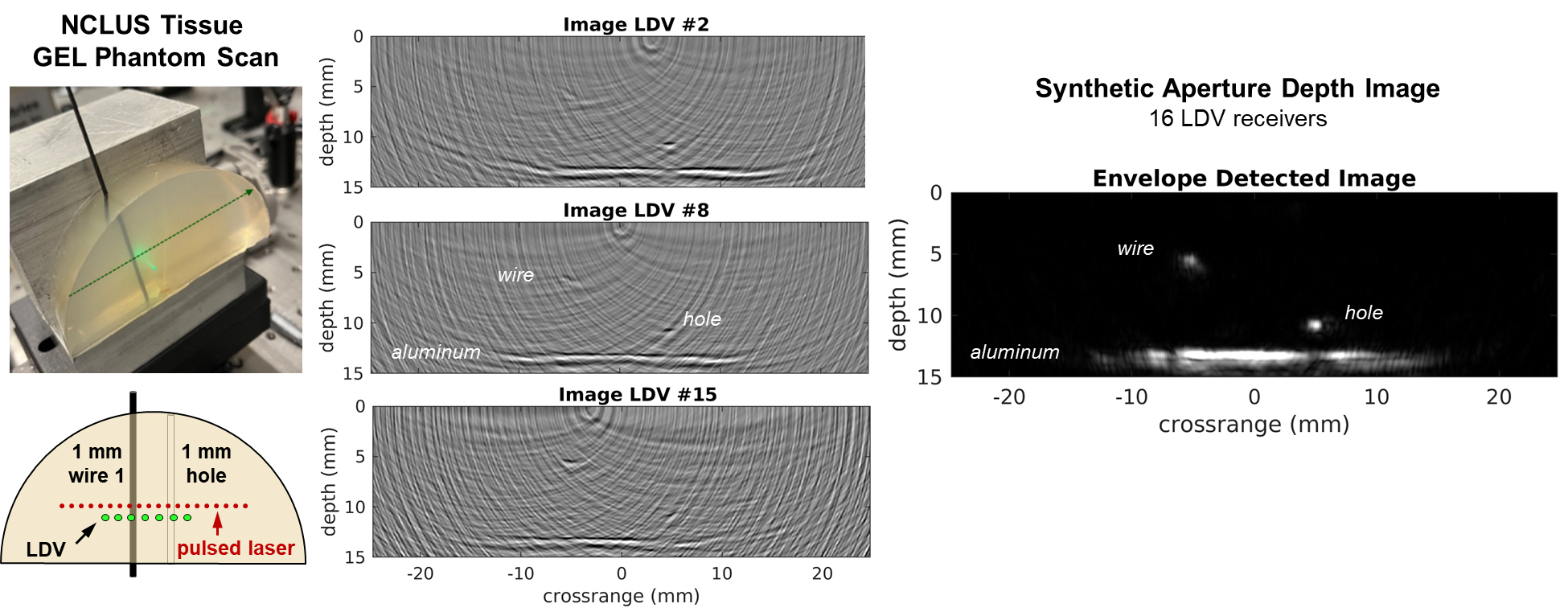
The team first demonstrated the clinical NCLUS system on a gel-based puck synthesized to match the mechanical properties of human tissue (referred to as a phantom) that control ultrasound wave propagation. Testing with medical imaging phantoms helps ensure that systems and techniques for imaging the human body are operating correctly.
Through sponsored programs, the team is now developing NCLUS to support applications requested by the U.S. Department of Defense. These applications include detecting and characterizing life-threatening injuries from internal bleeding in organs; monitoring debilitating musculoskeletal injuries and their healing over time; and providing elastographic imagery of soft tissue and bone of amputee limb regions to accelerate the design and fitting of prosthetic sockets. Civilian-wise, NCLUS could similarly be used to assess work, sports, and accident-related injuries and cases in the intensive care unit. With NCLUS, emergency medical technicians, paramedics, and medical staff without specialized sonography training might be able to perform ultrasound imaging outside of a hospital — in a doctor’s office, at home, or in remote settings such as on the battlefield or in rural locations where medical care may be difficult to access.
"With further development, NCLUS has the potential to be a transformative technology: an automated, portable ultrasound platform with a fixed-reference-frame capability similar to that of MRI and CT," Samir says.
In the next phase of the NCLUS program, the team will pursue clinical studies using an operational skin-safe laser to evaluate ultrasound images and compare them to those of conventional medical ultrasound. If these studies are successful, the team will seek commercial funding for clinical medical device development, followed by U.S. Food and Drug Administration agency approval.
This work is funded by the U.S. Army Military Operational Medicine Research Program. The human in vivo testing was approved by the MIT Committee on the Use of Humans as Experimental Subjects.
Illustrations and ultrasound images were supplied by Robert Haupt.
Inquiries: contact Ariana Tantillo.
Related Links
- Nature Light: Science and Applications paper, "Full noncontact laser ultrasound: first human data"
- Patent, "Systems and methods for generating non-contact ultrasound images using photoacoustic energy"
- Patent, "System and method for non-contact ultrasound with enhanced safety"
- Patent, "System and method for analyzing tissue using shear waves"
- MIT News, "Researchers produce first laser ultrasound images of humans"